Mechanochemistry – Argument for Brute Force
Mechanochemistry is a lesser-known but rapidly advancing field in chemistry. It challenges the traditional methods of activating chemical reactions, such as thermochemistry and electrochemistry, by introducing mechanical force as a powerful alternative. Aspects of mechanochemistry include macroscopic phenomena to single-molecule studies. This report covers both the theoretical and experimental aspects of mechanochemistry.
The report showcases how mechanical energy can directly induce chemical changes by breaking and reforming chemical bonds. Traditional chemistry textbooks seldom mention mechanochemistry as a viable method. The authors provide numerous examples, such as the cleavage of polymer chains under mechanical stress and the formation of new bonds in metals and alloys. They also use advanced techniques like atomic force microscopy (AFM) and molecular dynamics simulations to study single-molecule interactions provides novel insights that were previously inaccessible with conventional methods.
This has an impact on materials science and solid-state chemistry. The novel information presented includes the identification of specific conditions under which mechanical forces can outperform thermal or photochemical methods, such as in the controlled polymerization processes or the formation of unique nanostructures. Thus, mechanochemistry can be really relevant in various industries, from pharmaceuticals to materials engineering, where it could lead to more energy-efficient and environmentally friendly processes.
For follow-up research, it would be valuable to explore the applications of mechanochemistry in biochemistry, particularly in the study of proteins and nucleic acids under mechanical stress, and the development of new materials with enhanced properties, achieved through controlled mechanochemical reactions.
Beyer, M. K., & Clausen-Schaumann, H. (2005). Mechanochemistry: The Mechanical Activation of Covalent Bonds. Chemical Reviews, 105(8), 2921-2944.
Introduction to the Study:
The study focuses on how mechanical forces can activate chemical reactions, a process known as mechanochemistry.
Unlike traditional methods that use heat, light, or electricity to start reactions, mechanochemistry uses physical force. This offers a new way to carry out chemical reactions that could be more energy-efficient and environmentally friendly.
The report explores different examples of mechanochemistry, from breaking down polymers to forming new metal alloys. It has potential applications in many fields, including materials science, pharmaceuticals, and nanotechnology.
Methodology and Experimental Procedures:
Single-Molecule Studies: Researchers use advanced techniques, such as atomic force microscopy (AFM), to study the effects of mechanical forces on individual molecules.
Molecular Dynamics Simulations: These simulations help predict how molecules behave under mechanical stress, providing insights into their stability and reaction pathways.
Principles of Chemistry Demonstrated:
Bond Cleavage and Formation: Mechanochemistry can break strong covalent bonds and form new ones under controlled conditions.
Energy Transfer: Mechanical forces can transfer energy directly to chemical bonds, making them break or form more easily.
Reaction Pathways: The study shows that mechanical activation can create unique reaction pathways that are not possible with traditional methods.
Key Findings:
Mechanical forces can break polymer chains and create free radicals, which are highly reactive species that can form new bonds.
In metals and alloys, mechanochemistry can lead to the formation of nanostructures with unique properties.
The study demonstrates that mechanical activation can be more selective and efficient than thermal or photochemical methods for certain reactions.
Future Research Directions:
Further studies could explore the use of mechanochemistry in biological systems, such as proteins and DNA.
Research could also focus on developing new materials using mechanochemical methods, particularly those with applications in nanotechnology and electronics.
There is a need to better understand the fundamental principles of how mechanical forces interact with chemical bonds at the molecular level.
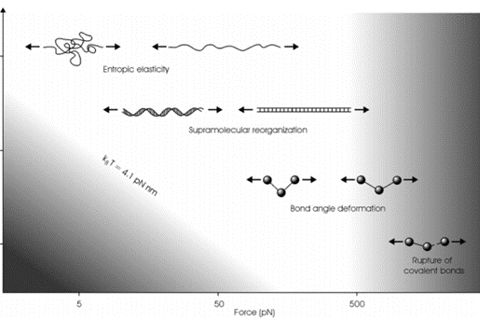
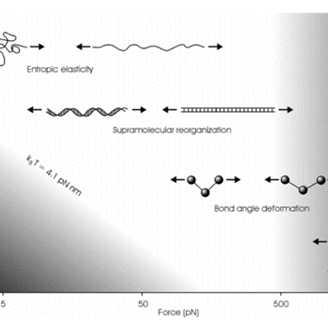
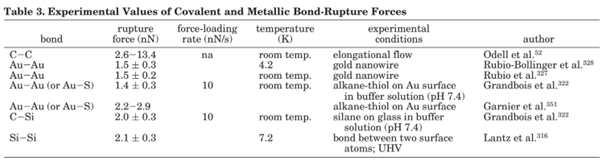
Accessible force range in Single molecule force spectroscopy. The thermal energy at room temperature sets the lower limit, while the upper limit is set the rupture force of the covalent bonds.