Aspirin – Synthesis and FITR Spectroscopy
As part of our A-Level lab work, I had to synthesize Aspirin from oil of wintergreen. I find the hands-on application that makes the learning experience more comprehensive, and this common experiment integrates both organic and inorganic chemistry principles, and highlights the conversion of a naturally occurring substance into one with therapeutic value. This paper presents another experiment on the synthesis, purification, and focuses on qualitative spectroscopic characterization of aspirin.
The article "Synthesis of Aspirin: A General Chemistry Experiment" by John Olmsted III provides an in-depth exploration of a redesigned experiment for first-year general chemistry students at California State University, Fullerton. This experiment focuses on synthesizing aspirin from oil of wintergreen, integrating both organic and inorganic chemistry principles, with the inclusion of a two-step synthesis process that begins with a common natural substance—oil of wintergreen. Most general chemistry experiments seem to involve simpler, one-step processes. The report explains that the multi-step synthesis not only introduces students to more complex chemical reactions which integrate a broader spectrum of chemical reactions, including hydrolysis and condensation, within a single experiment. Olmsted used this to teach students essential lab techniques like isolation, purification, and qualitative characterization using FTIR spectroscopy.
FTIR spectroscopy, which is becoming common in undergraduate labs, helps students distinguish between different compounds, such as salicylic acid and aspirin, through their unique spectral features.
Olmsted, J., III. (1998). Synthesis of Aspirin: A General Chemistry Experiment. Journal of Chemical Education, 75(10), 1261-1263.
Introduction to the Study:
The study focuses on the synthesis of aspirin (acetylsalicylic acid) as a key experiment in general chemistry education.
The experiment involves a two-step process: converting oil of wintergreen to salicylic acid and then synthesizing aspirin from salicylic acid.
The study introduces students to practical lab skills, including synthesis, purification, and spectroscopic analysis.
By teaching organic synthesis early, the experiment demonstrate the principles of chemical reactivity and stoichiometry used in both organic and inorganic chemistry..
Methodology and Experimental Procedures:
Step 1: Conversion of Oil of Wintergreen to Salicylic Acid:
Oil of wintergreen (methyl 2-hydroxybenzoate) is treated with a base (sodium hydroxide) to form salicylic acid.
The reaction involves hydrolysis, converting the ester into an acid.
The product is isolated by suction filtration and purified through recrystallization.
Step 2: Synthesis of Aspirin from Salicylic Acid:
Salicylic acid is reacted with acetic anhydride in the presence of a catalyst (phosphoric acid) to produce aspirin.
The reaction involves acetylation, adding an acetyl group to form the final product.
The aspirin is purified by recrystallization and characterized using FTIR spectroscopy.
Principles of Chemistry Demonstrated:
Hydrolysis and Condensation Reactions: The study illustrates two fundamental types of chemical reactions: hydrolysis (breaking down molecules using water) and condensation (joining molecules while releasing water)
Hydrolysis :HOC6H4CO2CH3 + H2O → HOC6H4CO2H + CH3OH
Condensation: HOC6H4CO2H+(CH3CO)2O→CH3CO2C6H4CO2H+CH3CO2H
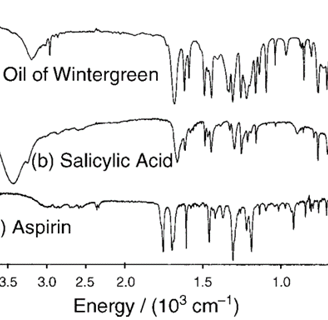
the hydrolysis reaction proceeds in several steps involving deprotonation and protonation as well as cleavage of a C–O bond (7). These encompass examples of Brønsted acid–base proton transfer, another major class of chemical reactionsSpectroscopic Analysis: The use of FTIR spectroscopy allows students to characterize the compounds and observe changes in functional groups throughout the synthesis.
Use of FTIR Spectroscopy: The FTIR spectra confirm the identity of the products, showing characteristic peaks corresponding to the functional groups in oil of wintergreen, salicylic acid, and aspirin.
The three compounds share spectral features due to their common framework, for example the aromatic C–H bending vibrations in the 600–800 cm-1 region. They differ substantially in the absorptions arising from the CO2H, CO2, and OH groups. Aspirin lacks the broad hydrogen-bonded OH absorption that is prominent in oil of wintergreen and salicylic acid between 3000 and 3500 cm-1. On the other hand, whereas oil of wintergreen and salicylic acid have a single C=O absorption at about
1700 cm-1, aspirin has two distinct peaks arising from its ester and acid C=O groups.